How Can AI Help Teachers? – An Interview with ChatGPT
March 22, 2023
Monthly Newsletter – April 2023
April 5, 2023Hydrogen is the lightest and most abundant element in the universe. That’s terrific news because, in both the present and the future, hydrogen is proving to be one of our greatest allies in developing green energy alternatives and combating climate change. Read more to understand hydrogen’s importance and how we can use it!
The Benefits and Importance of Hydrogen Fuel Cells
You’ve probably heard about hydrogen fuel cells before, either in a classroom setting, on the internet, or in the news. But what are they and how do they work? Before we get into that, let’s talk a little about what makes them so great.
Using hydrogen fuel cells to power the world around us comes with many benefits, both to us and our environment because:
- They run via chemical reactions (as opposed to combustion); they are extremely efficient.
- They work continuously as long as hydrogen fuel is supplied and provide instant power/torque and fast refueling, so they are reliable.
- They have no moving parts; they are quiet and rarely require maintenance or repair.
- Smaller units can be combined in series into large stacks, so they are scalable.
- Perhaps most importantly, they produce only heat, electricity, and water as they run; they are clean and safe for the environment.
One of the biggest applications of this technology that we’ve been seeing lately is hydrogen-powered cars, like the Toyota Mirai and the Hyundai Nexo. Considering the fact that sources of transportation (like cars, trucks, ships, trains, and planes) are one of the biggest contributors to greenhouse gas and carbon emissions that we release into the atmosphere, this is a step in the right direction and more important than ever. Scientists and engineers continue to work diligently on improving the cost, efficiency, and effectiveness of hydrogen-powered vehicles in order to make their further implementation a reality.
Sources of Hydrogen Gas
In order to run a hydrogen fuel cell, we first need to collect some of its fuel: hydrogen gas. By itself, pure hydrogen gas exists in a diatomic, gaseous form (H2). Because hydrogen gas in this form is so light, much of it simply rises into Earth’s upper atmosphere and eventually escapes into space. (It’s for this reason that hydrogen gas makes up a mere ~0.00005% of Earth’s atmosphere.) But fortunately for us, hydrogen exists in abundance on our planet in forms where it is “locked in” and combined with other elements, in molecules like water and hydrocarbons. There are three primary ways to extract hydrogen from such compounds:
- Steam-Methane Reforming: In this process, methane (CH4) is allowed to react with extremely hot steam (H20) in a low-pressure environment. This reaction allows the elements involved to recombine and form hydrogen gas (H2), carbon monoxide gas (CO), and carbon dioxide gas (CO2). This process currently accounts for over 95% of the world’s hydrogen production. The only problem with this method? It creates greenhouse gasses as a byproduct and requires a considerable amount of energy to get started (and usually that energy comes from fossil fuels, thus more greenhouse gas emissions). Not exactly the greenest option.
- Biological Processes: Hydrogen gas can also be harvested as the product of biological processes like decomposition, fermentation, and other metabolic processes performed by microorganisms like bacteria and algae, but these processes also tend to release greenhouse gasses into the atmosphere.
- Electrolysis: Yet another way to create hydrogen gas is to electrify water to separate its hydrogen atoms from its oxygen atoms. The beauty of this method? It does not directly produce any greenhouse gases or carbon emissions. However, this method is only as green as the source of electrical energy used to drive the process in the first place. For this reason, if we want to keep things green, it’s key to generate this electrical energy via green energy sources, with wind and solar being the cleanest.
Electrolysis: Creating Hydrogen Gas from Water
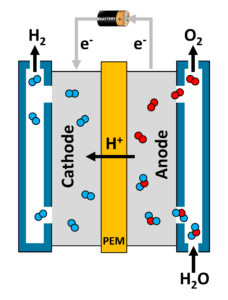 Today we’ll be focusing on the potentially greenest of the methods listed above: electrolysis. The process of electrolysis first begins by introducing water to a device called an “electrolyzer.” As the water molecules enter the device, a voltage is applied between the fuel cell’s anode and cathode using an external power source. This coaxes the molecules of water (H20) to split, forming hydrogen ions (H+, aka protons), electrons (e–), and diatomic molecules of oxygen gas (O2).
Today we’ll be focusing on the potentially greenest of the methods listed above: electrolysis. The process of electrolysis first begins by introducing water to a device called an “electrolyzer.” As the water molecules enter the device, a voltage is applied between the fuel cell’s anode and cathode using an external power source. This coaxes the molecules of water (H20) to split, forming hydrogen ions (H+, aka protons), electrons (e–), and diatomic molecules of oxygen gas (O2).
At this point, our hydrogen ions pass through a portion of the electrolyzer called a “proton exchange membrane,” or PEM for short. The electrons that were stripped from our hydrogen atoms go for a ride through the external power source mentioned previously. Our oxygen gas is now free to exit the electrolyzer, to be collected for later use or to simply be released into the atmosphere.
Once our hydrogen ions reach the other side of the PEM, entering the cathode region of the electrolyzer, they are gleefully reunited with their long-lost electrons, forming hydrogen gas that can be used to run a hydrogen fuel cell! The key thing to remember here is that we need to apply energy via our external power source to make this whole process of electrolysis occur. The greener that source of energy, the better.
Running a Hydrogen Fuel Cell
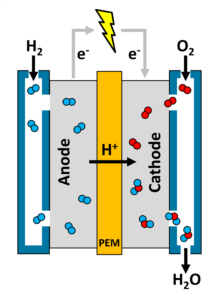 The process of running a hydrogen fuel cell is very similar to the process of electrolysis, just in reverse. In fact, many kinds of hydrogen fuel cells are designed to be “reversible fuel cells” that can function as both the electrolyzer and the fuel cell.
The process of running a hydrogen fuel cell is very similar to the process of electrolysis, just in reverse. In fact, many kinds of hydrogen fuel cells are designed to be “reversible fuel cells” that can function as both the electrolyzer and the fuel cell.
First, hydrogen gas is introduced to the anode of the fuel cell and splits to form hydrogen ions and electrons. The hydrogen ions pass through the PEM while the electrons pass through a circuit connecting the anode to the cathode. (The current generated by the flow of these electrons is what allows a hydrogen fuel cell to power something like a hydrogen-powered car!) On the other side of the PEM, in the cathode region of the fuel cell, our hydrogen ions, electrons, and oxygen gas react together to form clean, pure water. Behold, electricity from hydrogen!
Live In Action at ITEEA
 If you’re interested in learning more about hydrogen fuel cells and are planning on attending this year’s annual ITEEA conference in Minneapolis, be sure to sign up for my April 12th workshop entitled “Exploring Renewable Energy and Hydrogen Fuel Cells with Project-Based Learning.” In this workshop, attendees will have the opportunity to create their own miniature, hydrogen-powered kart using recyclable materials and a small reversible hydrogen fuel cell! Participants will leave with their own functioning hydrogen-powered kart and some ideas for applications in the classroom.
If you’re interested in learning more about hydrogen fuel cells and are planning on attending this year’s annual ITEEA conference in Minneapolis, be sure to sign up for my April 12th workshop entitled “Exploring Renewable Energy and Hydrogen Fuel Cells with Project-Based Learning.” In this workshop, attendees will have the opportunity to create their own miniature, hydrogen-powered kart using recyclable materials and a small reversible hydrogen fuel cell! Participants will leave with their own functioning hydrogen-powered kart and some ideas for applications in the classroom.
If you can’t make it to ITEEA this year, no worries. In lieu of experiencing the magic of hydrogen fuel cells in person, you can always check out the Horizon Educational products featured on our website! These kits are a great way to immerse students into the world of renewable energy with hands-on, project-based learning in mind.
Until next time, folks.
– Dr. Jake Roark